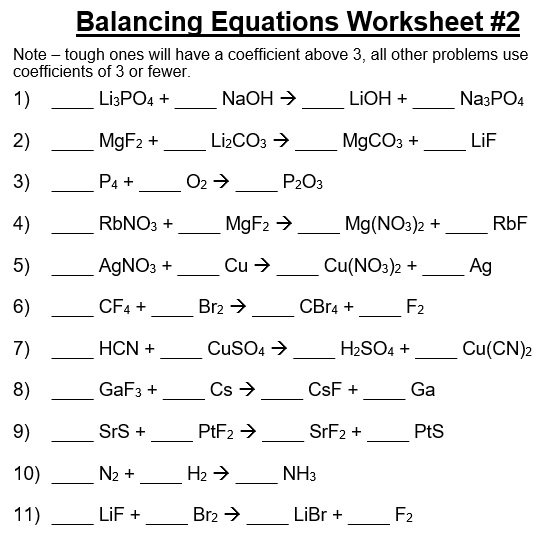
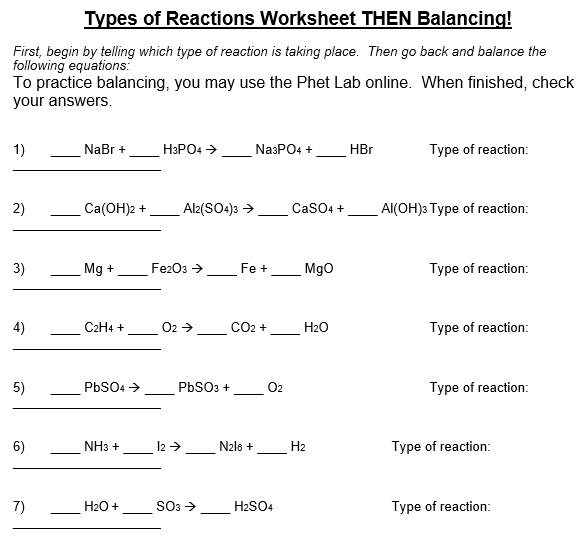
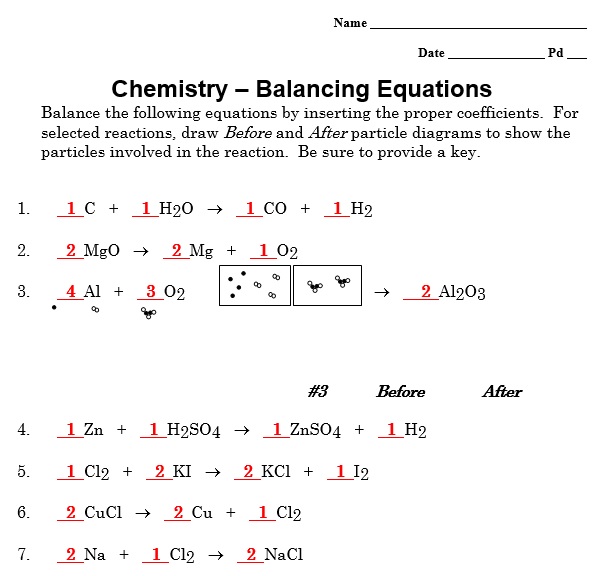
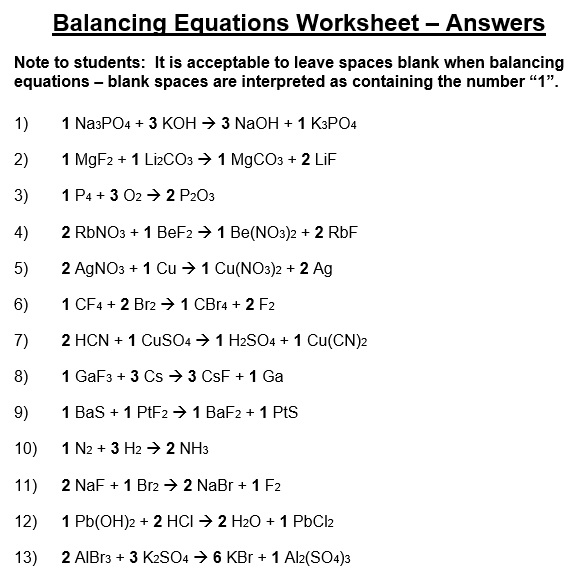
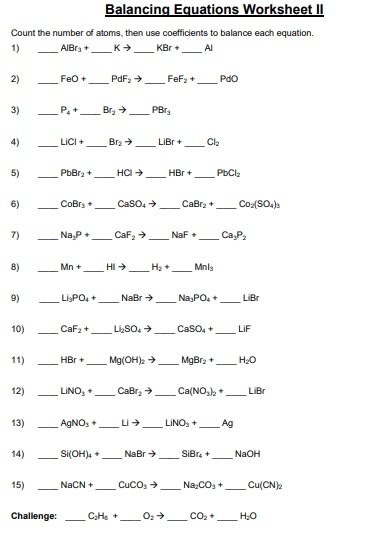
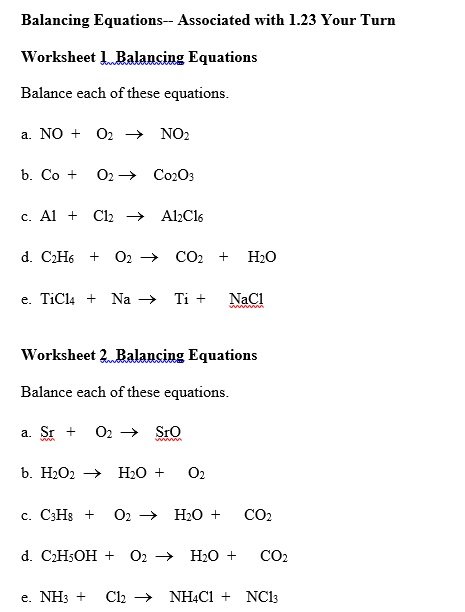
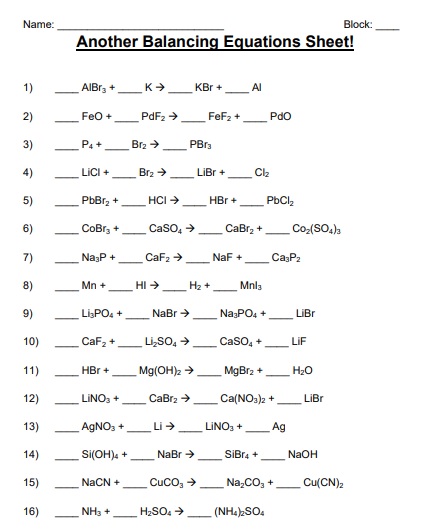
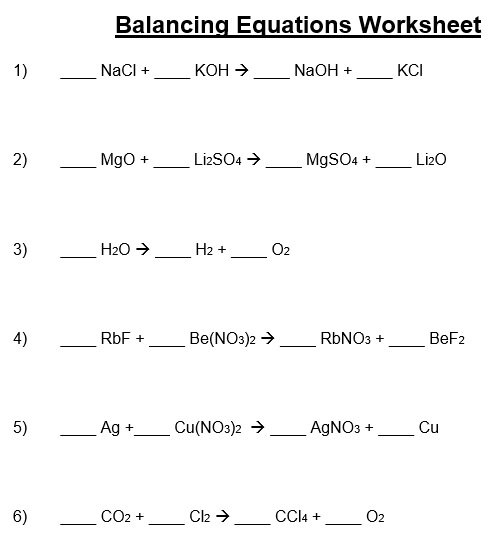
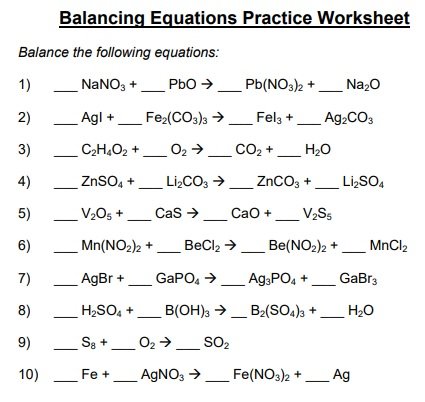
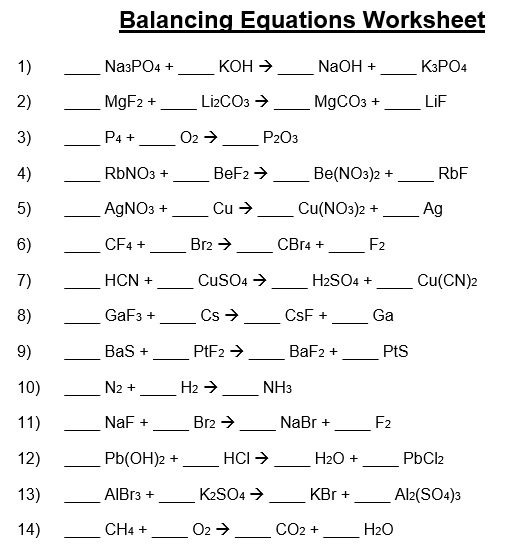
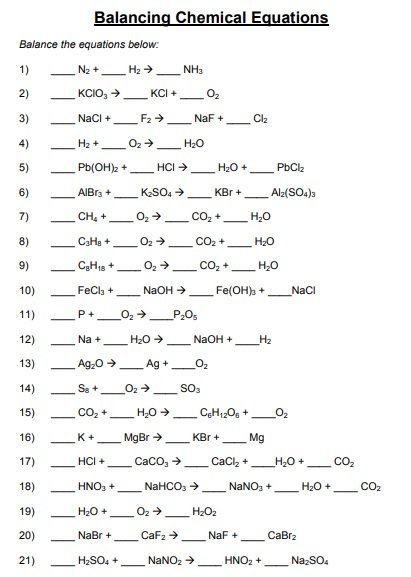
A balancing chemical equations worksheet is given to the students to balance the chemical equations. Many students find it difficult to balance equations. Balancing requires a lot of different techniques, formulas, symbols, and knowledge of reactions. During balancing equations, many students lose hope and struggle to solve it then they need balancing chemical equation worksheet with answers.
Table of Contents
- 1 What is a chemical equation?
- 2 Why balancing the chemical equation is important?
- 3 Different types of chemical equations:
- 4 How to balance a chemical equation?
- 5 Some tips for balancing chemical equations:
- 6 Two methods Use for balancing chemical equation:
- 7 What are the limitations of chemical equation?
- 8 Difference between balanced and unbalanced chemical equations:
- 9 What are the fundamental aspects of a balanced chemical equation?
- 10 Conclusion:
What is a chemical equation?
In Chemistry, a chemical equation represents chemical reaction with the help of chemical formulas. It contains chemical substances, reactants, and products that are involved in the reaction. In a chemical reaction, the reactions are the elements that react with one another and the elements that we get over the reaction are known as products. Moreover, the reactants are written on the left side of the chemical equation. While the products are on the right side of the chemical equation.
The balance chemical equation must contain an equal amount of reactants and products. In the balancing chemical equation worksheet, when students get big chemical equations, they find it very difficult to solve. You may also like Genogram Templates & Symbols.
Why balancing the chemical equation is important?
Sometimes you stuck in balancing chemical equations and you may wonder why you are doing so. Some students don’t bother it, but some try being logical to know the actual reason behind balancing it. Balancing is necessary because there must be an equal number of reactants and products on both sides of the equation.
Furthermore, according to the law of the Conservation of Mass, there must be an equal number of atoms on both sides of the equation. In 1789, Antoine Laurent developed this law and explored that the matter either can’t be destroyed or created. Also, unequal equations are not correct equations they have to be balanced properly. In calculating the chemical reactions, the unbalanced equations can’t be used.
Additionally, chemicals will not react until you have added the correct mole rations. Also, the balanced chemical equation informs you how much reactant you would need to have for making particular products. It means that you can’t get the correct products until you add the right amount of reactants.
Different types of chemical equations:
There are five different types of chemical equations. Let us discuss them below;
Synthesis Chemical Reaction:
It is the most common type of chemical equation in which by combining two to three combinations of reactants a new product is formed. For example, in H2+O→H2O, in this equation two atoms of hydrogen and one atom of oxygen combine to form a product, water. Therefore, it is known as synthesis reaction. It is an unequal equation because the number of reactants is not equal to number of products. You can balance the equation by using a combustion method.
Decomposition Chemical Reaction:
In this type of chemical reaction, only one compound undergoes the chemical reaction and results in two or more than two products. For example, Pb(No3)2→PbO + NO2 + O2, in this equation lead nitrate breaks down to form nitrogen dioxide, oxygen, and lead oxide.
Displacement Reaction:
This type of chemical reaction consists of two types i.e. Single displacement and double displacement. In single displacement chemical reaction, only one chemical partner exchanges from reactants to products. XY + Z → XZ + Y is an example of the single displacement reaction.
While, in double displacement reaction, two sets of chemical partners exchange from reactants to products. BaCl2 + NaSO4 → BaSO4 + 2NaCl would be an example of double displacement.
Combustion reaction:
When an oxygen compound and carbon compound combine together to become H2O and CO2, this reaction is called combustion reaction. In this type of reaction, only organic compound such as oxygen burns yielding to water, carbon dioxide, etc. In short, when oxygen combines with any substantial results in combustion.
Acid base reaction:
Acid base reaction is also known as neutralization reaction is a simple reaction where acid and base are combined together to form water and salt. Such type of reactions occurs usually in biological systems.
How to balance a chemical equation?
If you find difficulty in balancing chemical equations, then follow the below-mentioned steps;
Write down the balance equation:
At first step, write down the chemical formula of the reactants on the left side of the equation. Then, write down the products on the right side of the equation. There is an arrow between the reactants and products which indicates the direction the reaction is happening in. Now, you have all the unbalanced data it will help you in balancing the equation.
Balance the equation:
Now, you have to apply the law of conservation of mass. It states that equal numbers of atoms should be present on both sides. First look for an element that has only one reactant and produced. This is one of easiest ways to balance chemical equations. After balancing an element, move towards balancing the other one. In this way, you can balance all the elements.
You can balance the chemical formula by placing the co-efficient. Sometimes people get confused and add subscripts. By doing this, they totally change the formula.
Mention the states of matter:
Mention the states of matter of the products and reactants. Use g for gaseous substance, l for liquid substance, and s for solids. You should also check Lesson Plan Templates.
Some tips for balancing chemical equations:
Consider the following tips for accurately balance chemical equations;
- Keep in mind while balancing chemical equations that you can only modify the value of coefficient in front of the element or compound not the subscript.
- Another important thing to remember is that polyatomic ions have to be balanced as a whole.
- The number that has the greatest number of atoms in any product or reactant, you should balance them first.
- On both sides, count the number of atoms of each element and then check whether or not the equation is balanced.
- Make sure to check the co-efficient when you successfully balance the equation.
Two methods Use for balancing chemical equation:
Combustion method:
This method contains oxygen on both sides. Sometimes they are difficult to balance. You can easily balance the equation by missing a fraction of ½. But you can’t have a fraction for the coefficient so you should double all the coefficients. This will help you in balancing the equation.
Proportion method:
This is the second method used when the chemical equation is difficult to inspect. Make sure that you have to change coefficients not subscript.
What are the limitations of chemical equation?
Here are some limitations for chemical equations;
- Some chemical equations can’t clarify the state of substances that’s why, you have to add g for gas, l for liquid, s for solid and vap for vapor.
- The chemical equation doesn’t provide any details about the speed of reaction.
- Sometimes, terms like concentrated and diluted are used because the chemical equation doesn’t provide the concentration of the substances.
- It also doesn’t provide any details regarding the speed of the reaction.
- There is a diverse effect of some chemical equations and reactions.
Difference between balanced and unbalanced chemical equations:
In an unbalanced chemical equation, the mass and charge of the reactants and the products aren’t equal. It doesn’t indicate the amount required for the reaction to observe the conservation of mass.
In a balanced chemical equation, the mass and charge of the reactants and the products are equal. That’s why, it satisfies the law of conservation of mass.
What are the fundamental aspects of a balanced chemical equation?
The fundamental aspects of a balanced chemical equation are;
Reactants
It is any substance present at the start of a chemical reaction and placed on the L.H.S. By the end of the reaction, it is expected to undergo a physical or chemical change.
Products
Any substance that comes as a result of a chemical reaction or a physical change. It is written on the R.H.S of a chemical equation.
Coefficients
A number which is used to indicate the relative amount in a chemical equation is referred as a coefficient. In the equation, it is placed in front of symbols or formulas in the equation.
Physical state
It is important to explain the physical state of a substance in a chemical equation either it is solid, liquid, gas, or aqueous.
Conclusion:
In conclusion, students find difficulty in balancing chemical equations worksheet. To resolve this issue, there are worksheets with answers on different websites. You can easily download them and cross check your chemical reactions. You can also follow the above steps for balancing the chemical equation.